Comprehensive IGCSE States of Matter Notes | CKM Academy
Understanding the states of matter is very fundamental to understanding many of the key ideas in chemistry. Whether you’re revising for your IGCSE exams or just want to get a thorough grasp of the topic, this guide will take you through all the key points. This guide covers everything from the IGCSE State of Matter Notes, and characteristics of solids, liquids, and gases to the more intricate details of kinetic theory.
Introduction to States of Matter
Matter is defined as anything that contains mass and occupies space. The state of matter refers to the physical state in which matter exists. These states vary in terms of particle arrangement and energy; therefore, a substance’s properties depend on them. In light of chemistry and the IGCSE syllabus, it is of paramount importance to understand the states of matter. This topic serves as an overview of more advanced subjects like the chemical bonding of matter, the chemical reactions between different forms of matter, and gases’ behaviour.
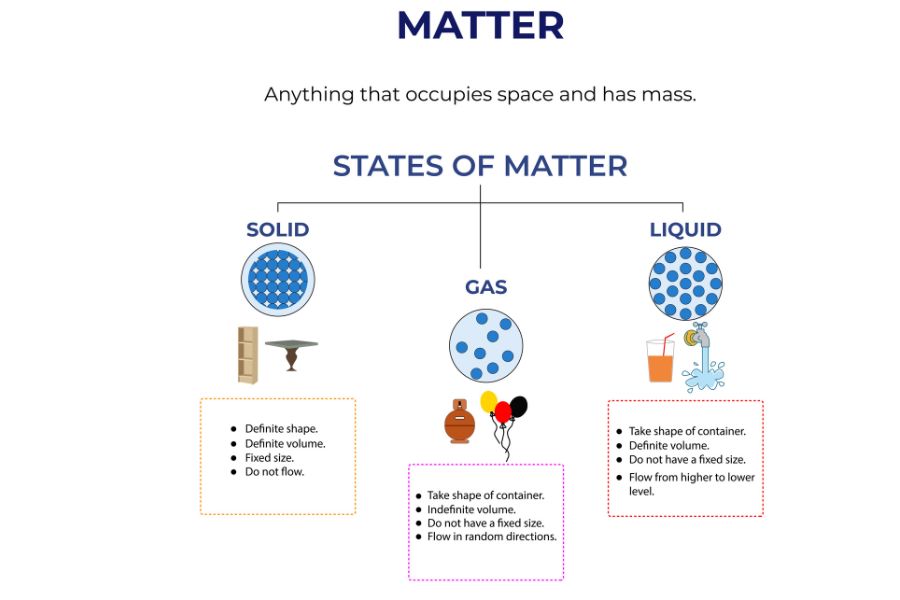
The three states of matter
Matter can occur in three traditional states: solid, liquid, or gas. Each state, or phase, has distinct properties that define and distinguish it from the other states.
Solids
Solids have a fixed shape and volume. The particles of a solid are very closely packed in a regular manner, so that they can vibrate without changing their relative positions. A solid essentially occupies an invariable volume due to its close packing.
Examples and Applications: Common examples of solids are ice, metals, and wood. Since everything that exists around us is a form of solid—from the chair we are sitting on to even the food that we consume—there exist an enormous number of forms of solids encountered in everyday life. The rigidity and structure of a solid are extremely important in many industries, including construction and manufacturing.
Liquids
Liquids possess a definite volume, while the container determines their shape. The particles are closer to each other but not as tightly packed as in a solid, so they will be able to move freely. This fluidity allows the liquids to flow and pour. At the same time, liquids are considered only slightly compressible since the particles are relatively close in the liquid state.
Examples and Applications: Examples of liquids include water, oil, and alcohol. In common life, liquids find application in everything from cooking to cleaning and transport—as in car engines. A major characteristic of liquids is their ability to take the shape of their containers, making them suitable for a wide range of uses.
Gases
Gases have neither a defined shape nor a defined volume. The gas particles are widely spaced and move freely, allowing gases to expand and fill their containers. Because the particles are widely separated, the forces of attraction between them are weak, and the gases are highly compressible. The energy of the gas particles is significantly higher than that of solids and liquids, primarily due to their greater mobility.
Examples and Applications: The common gases include oxygen, carbon dioxide, and nitrogen. Gases have a wide range of applications in both human and industrial processes. For instance, respiration uses oxygen, and carbonated drinks use carbon dioxide.
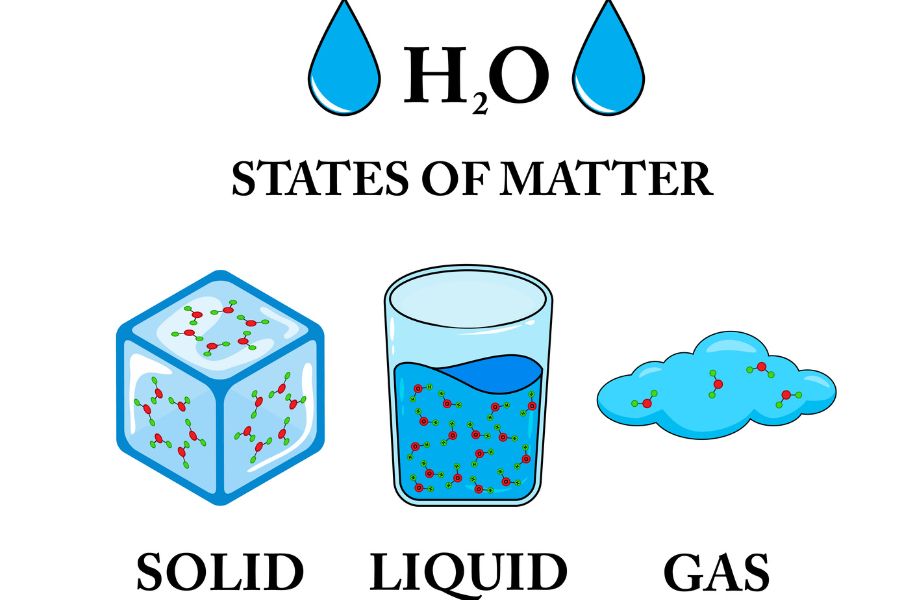
Changes of State
The addition or removal of energy can cause matter to change states from one state to the next. The changes of state include melting, freezing, boiling, condensation, sublimation, and deposition.
Melting and Freezing
Melting is the process where a solid turns into a liquid. Heat gives a solid’s particles enough energy to overcome their fixed positions. When heated above 0 °C, ice melts into water.
Freezing, on the other hand, is the opposite process, where a liquid becomes a solid. As a liquid cools, its particles lose energy and assume a fixed position. For instance, water freezes to ice at 0°C.
Condensation and boiling
Boiling occurs when a liquid transforms into a gas. Conditions lead to the liquid’s heating temperature reaching a specific level, known as the boiling point. At this point, it begins to acquire energy, and as a result, its particles have enough energy to break away from the liquid’s surface. Water at standard atmospheric pressure boils at 100 °C, changing into steam.
The reverse process, condensation, converts a gas into a liquid. Lowering the temperature of the gas causes the particles to lose energy and come closer together, forming a liquid. Example: Water vapour turns into droplets on a cold surface.
Sublimation and deposition
This is the less common change of state in which a solid becomes a gas without going through the liquid phase. An example of this is dry ice-solid carbon dioxide sublimating, turning directly into carbon dioxide gas.
Deposition is the opposite of sublimation. A gas will change directly into a solid without going through the liquid phase. Frost on a cold surface, formed from water vapor in the air, is an example of deposition.
Kinetic Theory of Matter
The kinetic theory of matter gives an insight into the behaviour of particles in their different states. All matter is considered to be composed of extremely small particles that are never at rest. The energy possessed by these particles, as well as the strength of the forces acting between them, determines the state of the matter.
- In solids, particles vibrate in fixed positions due to strong attraction forces.
- In liquids, the particles are free to move, though they remain close to one another, with forces of attraction that are still significant but weaker than in solids.
- In gases, particles move in a fast and far-apart motion, and forces of attraction are usually negligible.
In this way, the kinetic theory explains why solids have a definite shape, why liquids flow, and why gases fill up their containers.
Pressure and Temperature
Pressure and temperature are the two most important variables that influence a gas’s behaviour. Of the known laws that describe their relationship, two are the most important: Boyle’s Law and Charles’ Law.
- Boyle’s Law states that at constant temperature, the volume and pressure of a gas are inversely proportional. In other words, while a gas’s pressure increases, its volume decreases, or vice versa.
- Charles’s Law states that the volume of a gas is directly proportional to its temperature when the pressure is constant. This suggests that an increase in a gas’s temperature leads to its expansion, which in turn increases its volume, and vice versa.
Understanding these relationships is critical when trying out problems related to gases in chemistry on your own.
Practice Questions
1. Multiple Choice Question: Which one of the following changes of state involves the change in a gas to a liquid?
A) Melting
B) Boiling
C) Condensation
D) Sublimation
2.Short Answer Question: Explain the process of sublimation and give an example.
3.Extended Response Question: describe the kinetic theory of matter; use this theory to explain the properties of solid, liquid, and gaseous states of matter.
IGCSE State of Matter Notes—Source You Can Count On
The CKM Academy, Borivali West, has gained great experience and reputation in developing ideal IGCSE states of matter notes to ensure that students perform well in the examination. These notes include explicit and brief explanations of important concepts and detailed explanations of the properties and behaviour of solids, liquids, and gases, as well as their various state changes. Focused on the mission to make complex topics straightforward and understandable, CKM Academy provides students with all they may want to know to master this basic aspect of chemistry.
Ending Remarks
Basically, having mastery of IGCSE states of matter notes is important for understanding several concepts in chemistry. Effective revision involves:
- You should observe various properties and behaviours in solids, liquids, and gases.
- You can practice more by testing yourself on past papers and quizzes.
- This entails creating flashcards, diagrams, or flowcharts to help one memorize changes that occur in changing states of matter.
This way, if you follow these tips and study hard, you will be thoroughly ready for your IGCSE. Wishing you productive studying!
Image Reference: Freepik
Disclaimer: All trademarks, logos, and brand names are the property of their respective owners. All company, product, and service names used in this website are for identification purposes only. Use of these names, trademarks, and brands does not imply endorsement.