IGCSE Chemistry Periodic Table: Key Concepts and Exam Tips
Mastering the IGCSE Chemistry curriculum is very challenging for many students, especially when it comes to understanding the Periodic Table. However, this is the foundation on which all the other topics are built and, therefore, a very important one to understand well. The Periodic Table is not just a chart of elements; it is the key to unraveling the mysteries of chemical reactions, bonding, and the behavior of materials.
In this article, we will discuss expert tips and strategies that will help you understand the fundamentals of the Periodic Table . It does not matter whether you’re preparing for your exams or you just want to improve your skills in chemistry, this will help you understand the IGCSE Periodic Table and will give you confidence over your studies. Let’s get into the world of chemistry and discover the mystery behind this mighty tool
Introduction to the IGCSE Periodic Table
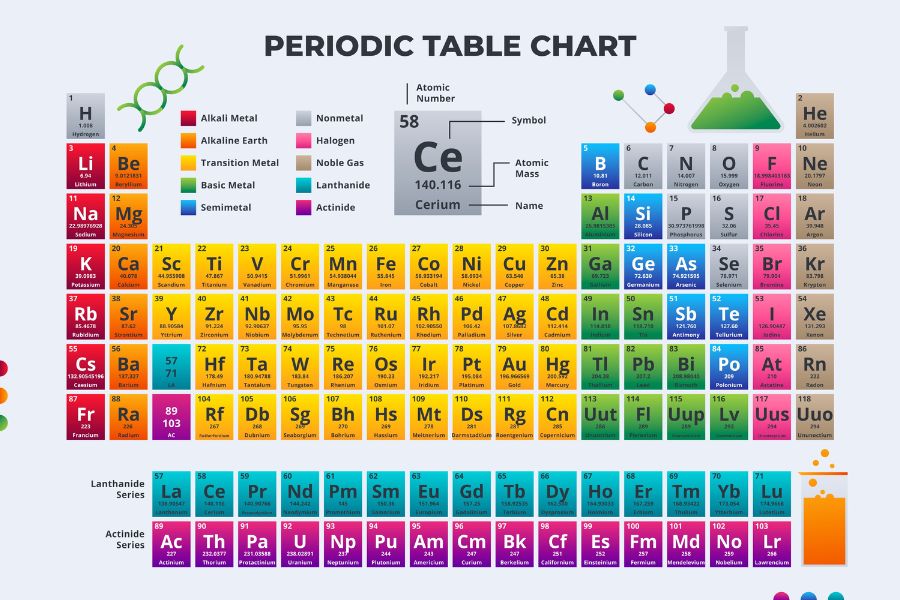
The IGCSE Periodic Table is more than just a chart filled with symbols and numbers. It’s a powerful tool that provides insights into the properties of elements and how they interact with one another. The IGCSE curriculum emphasizes the importance of understanding how elements are organized in the table, what their positions indicate about their chemical properties, and how these trends impact real-world chemistry.
Elements in the IGCSE Periodic Table are arranged in increasing order of atomic number. The rows are known as periods, while the columns are called groups. Each element’s position reflects its electron configuration and properties, including reactivity, electronegativity, and atomic size. The IGCSE Periodic Table becomes a key reference point for students as they progress through topics such as bonding, chemical reactions, and trends across the elements.
Structure of the Periodic Table
Understanding the structure of the IGCSE Periodic Table is crucial for Chemistry students. The table is divided into periods (horizontal rows) and groups (vertical columns). Periods represent elements with increasing atomic numbers and electrons filling the same electron shell, while groups contain elements with similar chemical properties due to having the same number of outer electrons.
- Groups: Elements in the same group, such as Group 1 (alkali metals) or Group 7 (halogens), have similar chemical behaviours because they share the same number of valence electrons.
- Periods: As you move across a period from left to right, elements have an increasing number of protons and electrons, which leads to trends in properties like electronegativity and ionization energy.
Key terms to familiarize yourself with include atomic number (the number of protons in the nucleus), mass number (the total number of protons and neutrons), and isotopes (atoms of the same element with different numbers of neutrons).
Periodic Trends and Properties
One of the most important aspects of the IGCSE Periodic Table is understanding the trends that occur as you move across periods and down groups. These trends help explain why certain elements behave the way they do and are frequently tested in IGCSE exams.
- Atomic size: As you move across a period, atomic size decreases due to the increasing nuclear charge pulling electrons closer to the nucleus. Moving down a group, atomic size increases as more electron shells are added.
- Electronegativity: This measures an atom’s ability to attract electrons in a bond. Electronegativity increases across a period and decreases down a group.
- Ionization energy: The energy required to remove an electron from an atom. Ionization energy increases across a period (due to stronger attraction between the nucleus and electrons) and decreases down a group (as electrons are farther from the nucleus).
Understanding these trends is key to predicting how elements will react with each other, an essential skill for IGCSE Chemistry students. The IGCSE Periodic Table simplifies this process by visually representing these trends.
Group-Specific Elements Overview
Each group in the IGCSE Periodic Table has distinct properties, and understanding these can help students predict chemical behaviour and reactions.
- Group 1: Alkali Metals
Group 1 contains highly reactive metals like lithium, sodium, and potassium. These elements have one valence electron, making them eager to lose that electron and form positive ions. As you move down Group 1, the metals become more reactive due to the increased distance of the outer electron from the nucleus. - Group 7: Halogens
The halogens, including fluorine, chlorine, and bromine, are highly reactive non-metals with seven valence electrons. These elements are eager to gain one more electron to achieve a full outer shell, making them strong oxidizing agents. Reactivity decreases as you move down the group. - Group 0: Noble Gases
Group 0 consists of noble gases like helium, neon, and argon, which are characterized by their full outer electron shells. This makes them chemically inert and unlikely to form compounds under normal conditions.
These group-specific properties are critical for predicting reactions, a key requirement in IGCSE exams.
Periodic Table and Chemical Bonding
The IGCSE Periodic Table is not just useful for understanding elements individually but also for predicting how they will bond with other elements. Elements tend to form bonds to achieve a stable electron configuration, usually similar to that of the noble gases.
- Ionic bonding: Typically occurs between metals and non-metals. Metals (like those in Group 1) lose electrons to form positive ions, while non-metals (like those in Group 7) gain electrons to form negative ions. These opposite charges attract, forming ionic bonds.
- Covalent bonding: Non-metals, especially those from Group 4 or Group 6, often share electrons to achieve a full outer shell. Covalent bonds are common between elements like carbon and oxygen.
The IGCSE Periodic Table aids in predicting which type of bonding is likely to occur based on the elements involved.
Common IGCSE Exam Questions on the Periodic Table
Exam questions related to the IGCSE Periodic Table often test students’ understanding of periodic trends, element groups, and bonding behaviour. Some examples of typical questions include:
- Predicting the reactivity of elements based on their position in the Periodic Table.
- Describing the trend in atomic size across a period or down a group.
- Explaining how the position of an element in the IGCSE Periodic Table affects its bonding type.
By practicing these kinds of questions, students can enhance their understanding and prepare effectively for their exams.
Where to Find IGCSE Chemistry Periodic Table Notes?
If you are looking for reliable and high-quality IGCSE Periodic Table notes, CKM Academy is an excellent resource. CKM Academy, located in Borivali West, is a well-established coaching centre that specializes in guiding students for IGCSE, A-Level, and IB courses. Founded by Ms. Chaitali Mehta in 2018, CKM Academy is known for offering a student-friendly approach, combining traditional teaching methods with modern technology to make learning interactive and enjoyable.
At CKM Academy, students can access IGCSE Chemistry Periodic Table notes, past papers, and other essential study materials. The Academy offers comprehensive notes that cover all aspects of the IGCSE Periodic Table, from understanding its structure and trends to mastering exam questions. By studying with CKM Academy, students gain the confidence and skills needed to excel in their IGCSE exams.
If you’re looking for personalized coaching and access to premium IGCSE Periodic Table notes and past papers, CKM Academy is an ideal choice.
Study Tips for Mastering the IGCSE Periodic Table
Mastering the IGCSE Periodic Table requires consistent practice and a strategic approach to memorization. Here are some tips to help you excel:
- Use mnemonic devices: To remember the order of elements in specific groups, create fun and memorable phrases.
- Create visual aids: Diagrams and colour-coded charts can help you visualize periodic trends and group behaviours.
- Practice with past papers: Solving past exam questions is one of the best ways to reinforce your understanding of the IGCSE Periodic Table and to familiarize yourself with the types of questions that may appear on the test.
Conclusion
At CKM Academy, we understand that the IGCSE Periodic Table is an essential resource for every IGCSE Chemistry student. It serves not only as a tool for organizing the elements but also as a guide for predicting their behaviour and reactions. Our expert faculty emphasizes the importance of grasping the structure, trends, and practical applications of the periodic table, ensuring you feel confident and prepared for your exams.
We provide comprehensive IGCSE notes and a wealth of past papers across all subjects to enhance your understanding and practice. Remember, regular practice with past papers and employing study strategies that prioritize understanding trends over rote memorization are key to your success. With the right preparation and resources from CKM Academy, you will excel in your IGCSE Chemistry journey!